Home > China Supplier Database > Chinese enterprise > Zhejiang Langhua Pharmaceutical Co.,Ltd
Enterprise Introduction
Zhejiang Langhua Pharmaceutical Co.,Ltd, formerly as Zhejiang Xinhua Pharmaceutical Co.,Ltd established in year 1986, is a U.S.FDA approved pharmaceutical company, focusing on Fluoroquinolone, Anti-virus , Cardiovascular APIs & advanced intermediates manufacturer in China.
We have been approved by FDA/WHO/EDQM/ANVISA for GMP, and granted with 1 EUGMP, 2 WHO-PQ GMP,2 Brazilian GMP certificates, 3 CEP certificates , 17 CFDA GMP certificates and 4 US-DMF.
Beside the ISO 14001&OHSAS18001 certificates, we have good EHS system which has been approved by governmental authorities and multi-national pharmaceutical companies.
We have professional and experienced R&D team constituted by Post-PhD, Doctors and Masters, over 10 years CMO experience, and dedicated pilot, scale-up workshop which complies with cGMP requirement. Can run ROS developing, lab trial, pilot, scaling-up, and commercial production, from Gram to Ton lot.
Certificates:
WHO-PQ: Zidovudine, Levofloxacin hemihydrate
EUGMP: Zidovudine
CEP: Zidovudine, Spironolactone, Enrofloxacin
ANVISA GMP: Zidovudine, Ciprofloxacin HCl
US-DMF:
Zidovudine, Spironolactone, Levofloxacin hemihydrates, Enrofloxacin
Enterprise dynamics more >
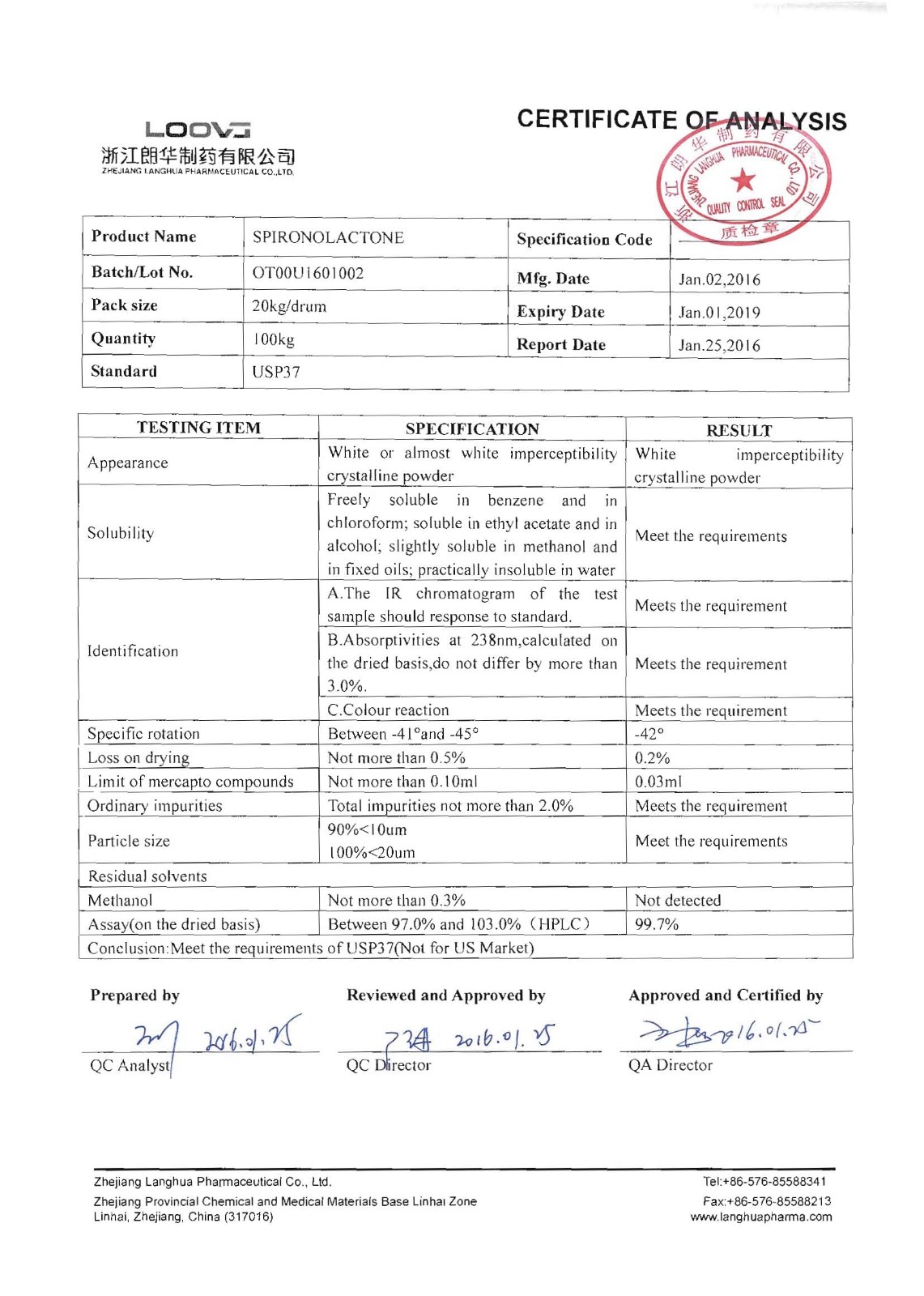
Spironolactone
Company name:Zhejiang Langhua Pharmaceutical Co.,Ltd
Location: Spironolactone
type:Cardiovascular system
Authentication: Not provided.
Release time: 2016/05/30
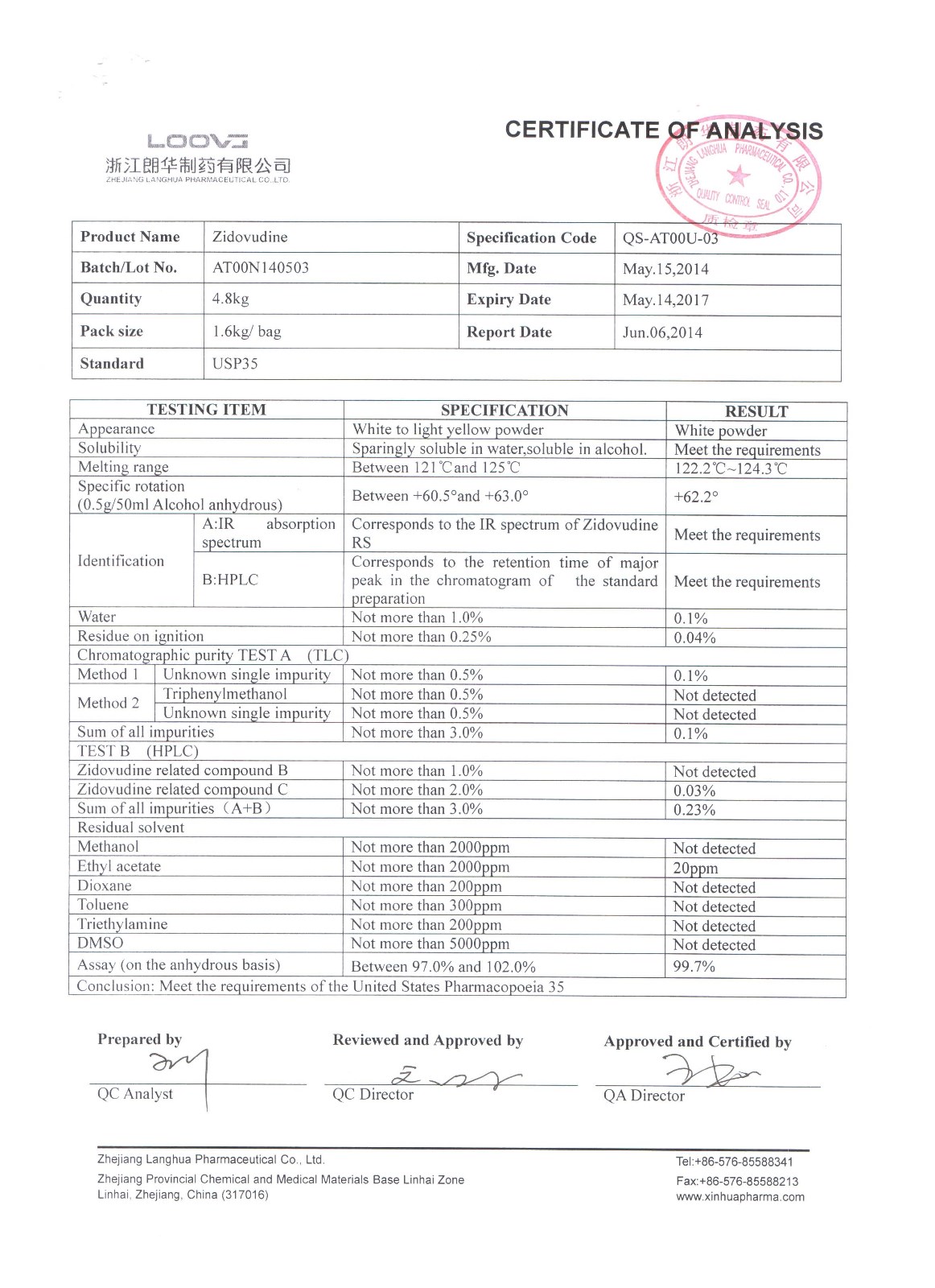
Zidovudine
Company name:Zhejiang Langhua Pharmaceutical Co.,Ltd
Location: Zidovudine
type:Other APIs
Authentication: Not provided.
Release time: 2016/05/30
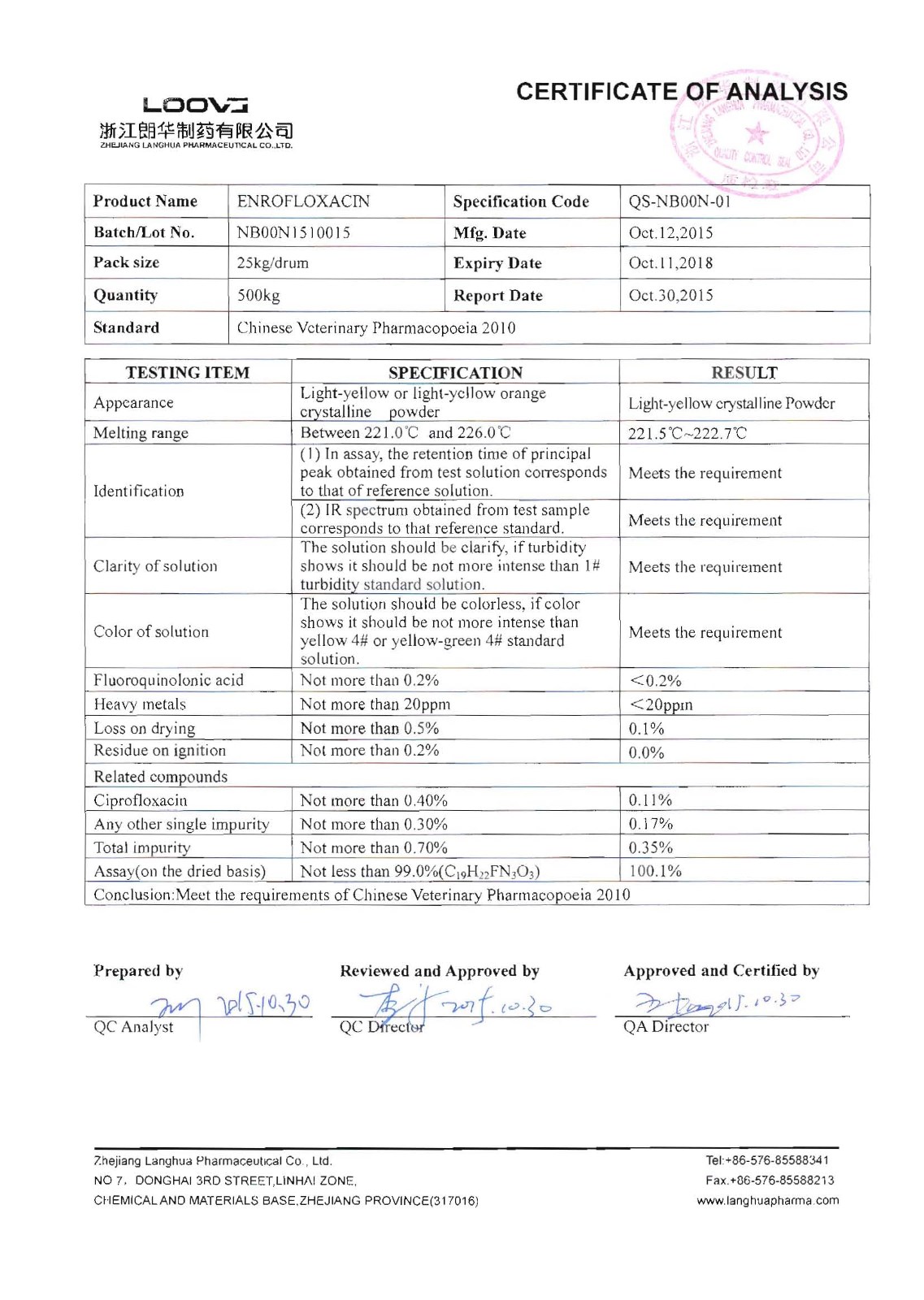
Enrofloxacin
Company name:Zhejiang Langhua Pharmaceutical Co.,Ltd
Location: Enrofloxacin
type:Anti-infectives
Authentication: Not provided.
Release time: 2016/05/30
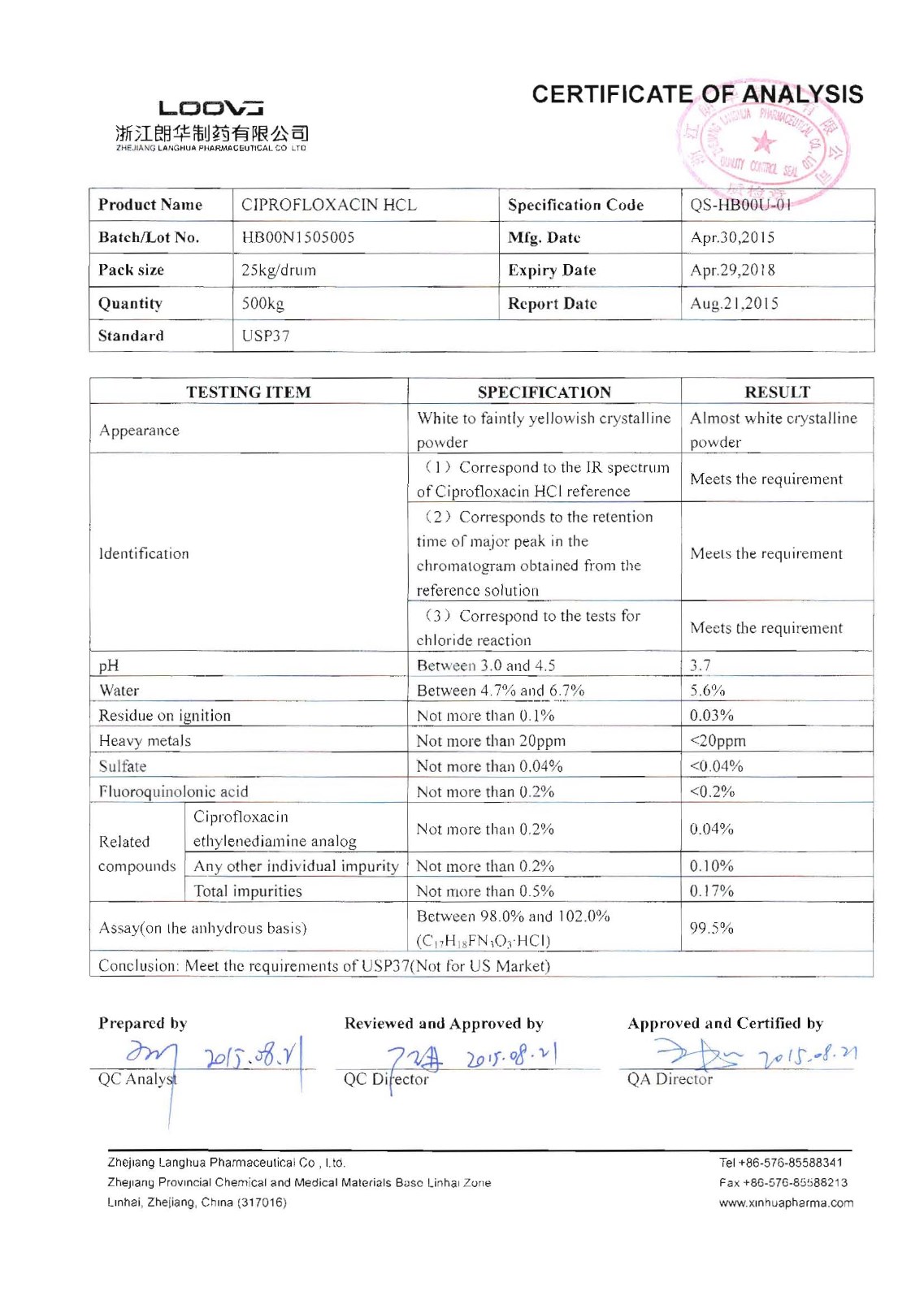
Ciprofloxacin Hcl
Company name:Zhejiang Langhua Pharmaceutical Co.,Ltd
Location: Ciprofloxacin Hcl
type:Anti-infectives
Authentication: Not provided.
Release time: 2016/05/30